ActivaNail™ -Absorbable Nail
ActivaNail is an advanced absorbable nail implant used in fixation of bone fractures, osteotomies, arthrodesis, and osteochondral fractures in the presence of appropriate immobilization. ActivaNail material is replaced by bone in approximately two years and eliminates the need for removal surgery.
Individual sterile-packaged and easy to insert with reusable instrumentation.
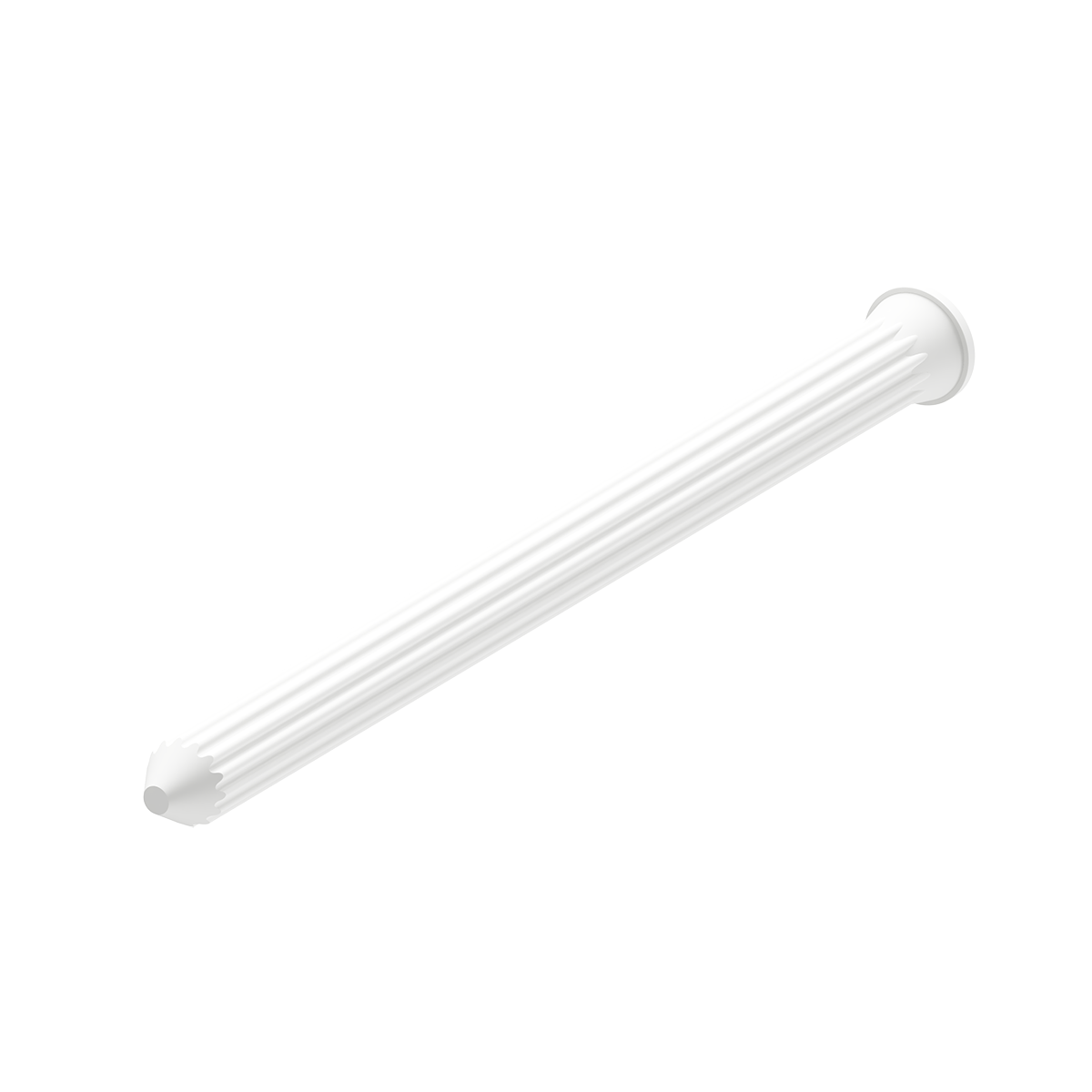
Enhances the rotational stability and provides higher strength with its patented grooved design and a conical head.
Excellent in pediatric orthopedics.
Strong track record of safe use.
For fixation of bone fractures, osteotomies, arthrodesis and osteochondral fractures of upper and lower extremities.
ActivaNail is an implant with excellent mechanical properties created by the tailored material and the proprietary manufacturing technique. It provides excellent strength retention over the bone healing period. The bending modulus of the material is close to the value of cortical bone which decreases stress shielding in bone.
ActivaNail utilizes Bioretec’s innovative Self-Locking SL™ technology and a grooved surface design with a conical head which reduces the risk of unstable fixation. This technology enables the implant’s diameter to expand 1-2% in the physiological environment, ensuring a secure and stable attachment to the bone.
Patient Benefits
Patented Self-Locking SL™ Technology
ActivaNail’s patented Self-Locking SL technology minimizes the risk of unstable fixation by enabling the implant’s diameter to expand by 1–2% in a physiological environment, creating a firm and secure bond with the bone.
Absorption Within Approximately 2 years
ActivaNail is absorbable and the implant degrades slowly in the span of approximately two years transferring the stress to the healing bone and assisting in the healing process.
Faster healing
The absorption of ActivaNail eliminates the need for removal surgery, reducing the risk of implant-related long-term complications and facilitating a smoother healing process.
Indication Examples of Activa Series
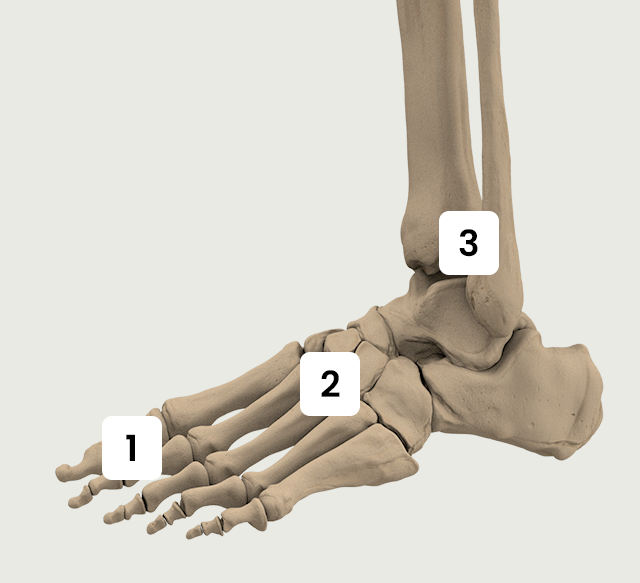
Foot & Ankle
The Activa series applies for fractures and orthopedic conditions in foot and ankle.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
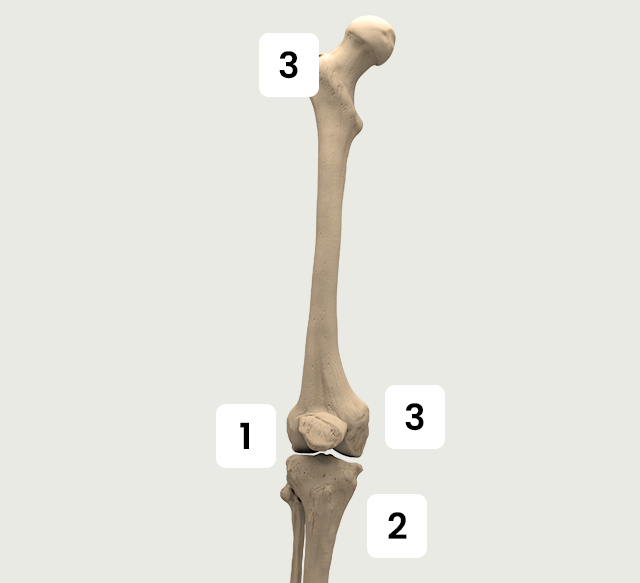
Knee
The Activa series applies for fractures and orthopedic conditions in knee.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
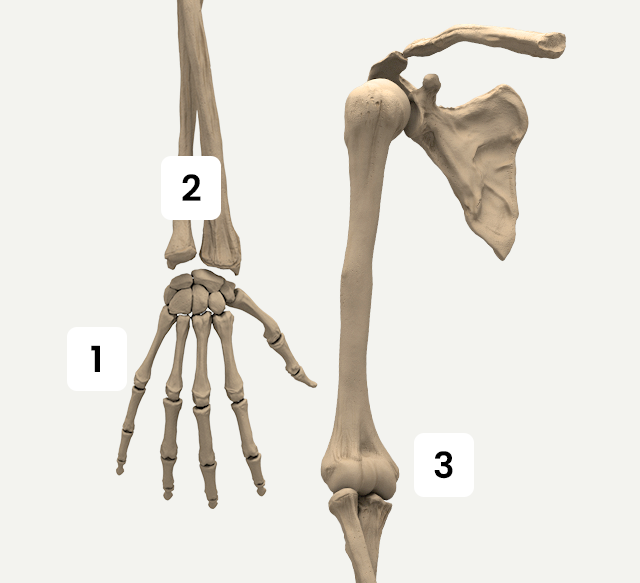
Upper Extremity
The Activa series applies for fractures and orthopedic conditions in Upper Extremity.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Pediatric
The Activa implants are clinically tested for over 20 years, are used in more than 40 countries, and have helped hundreds of thousands of adult and pediatric patients.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Activa -The Difference
Medial malleolus fracture of a 12-year old girl with absorbable ActivaScrew™ LAG
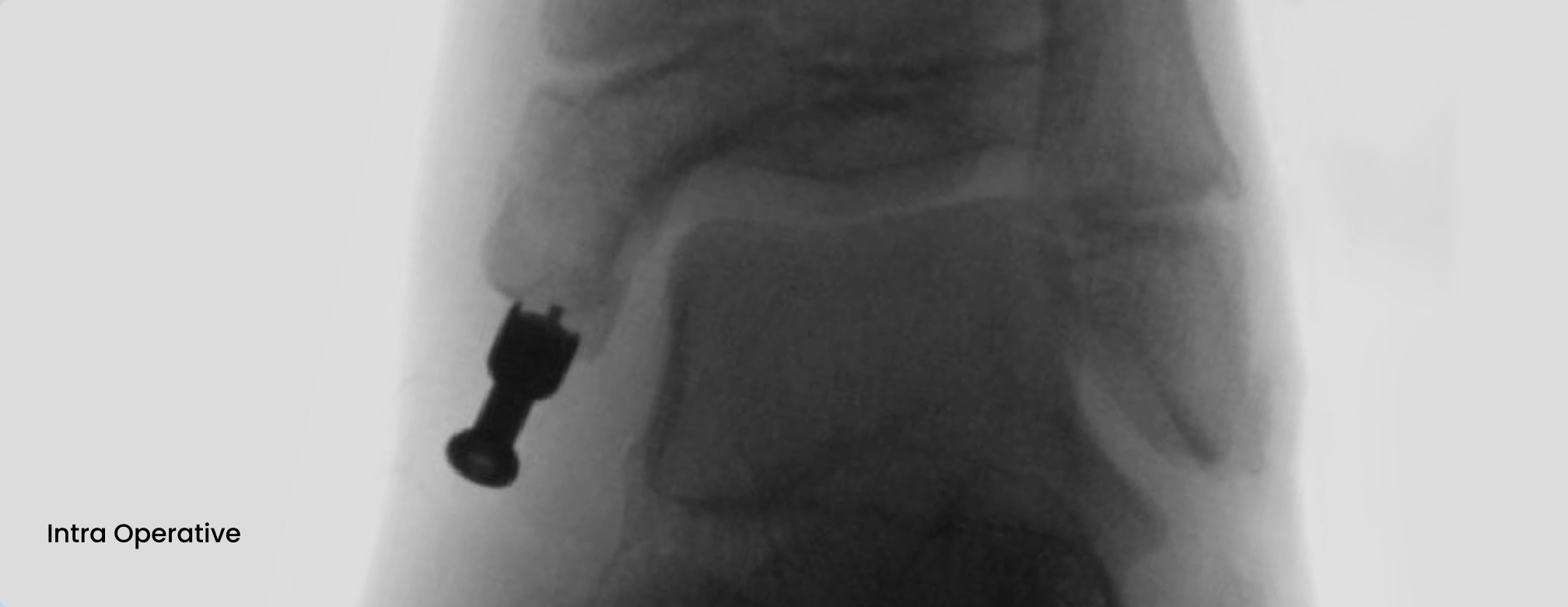
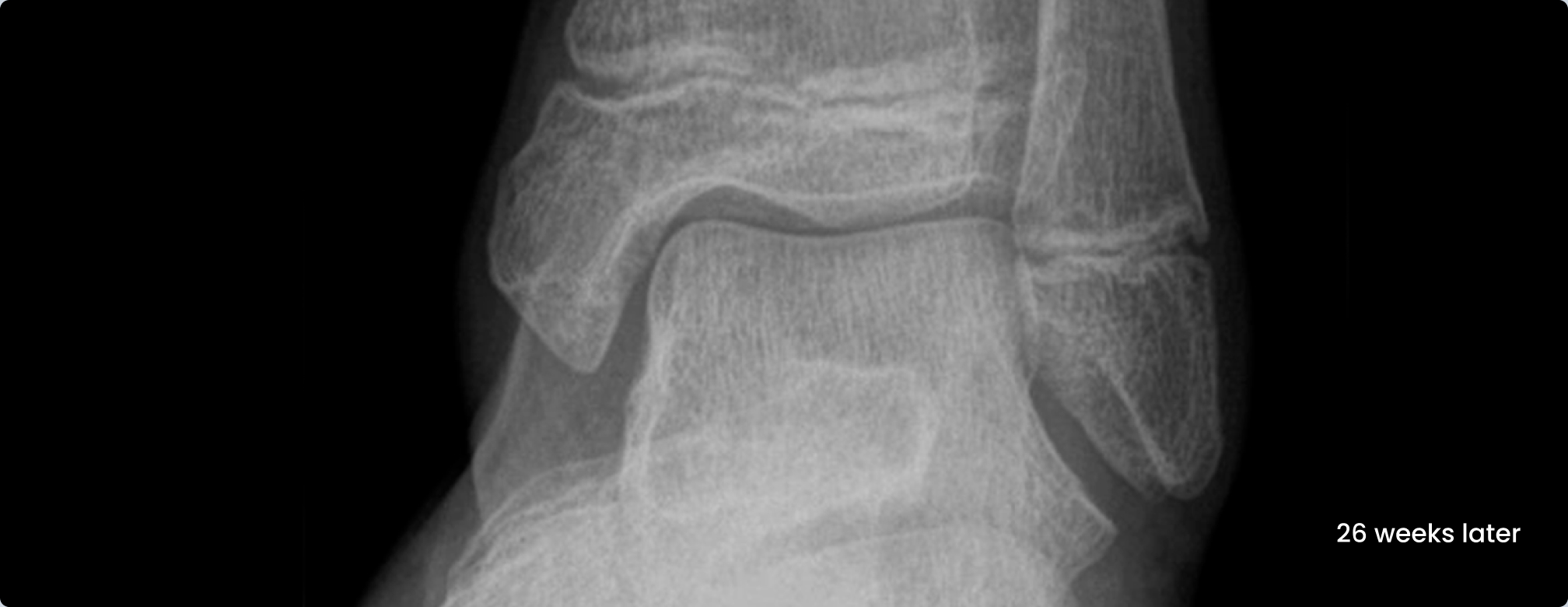
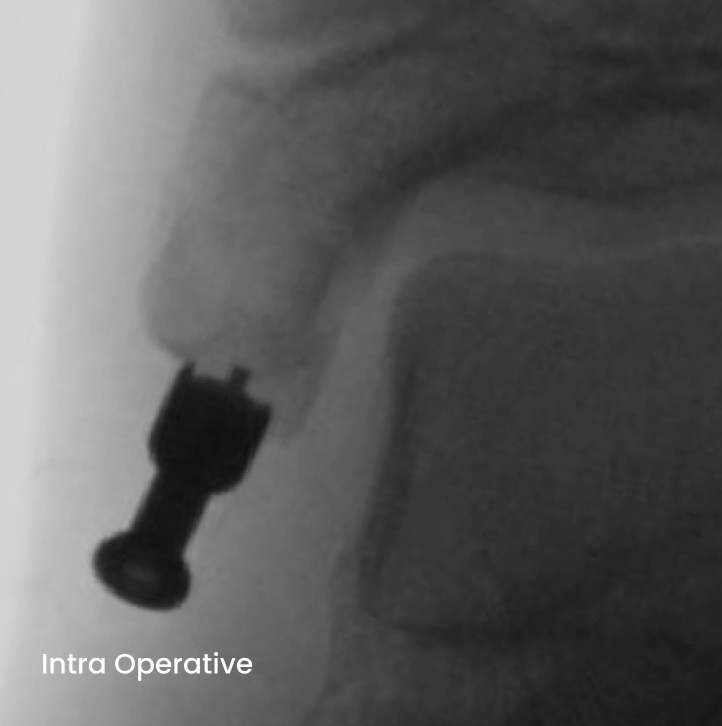
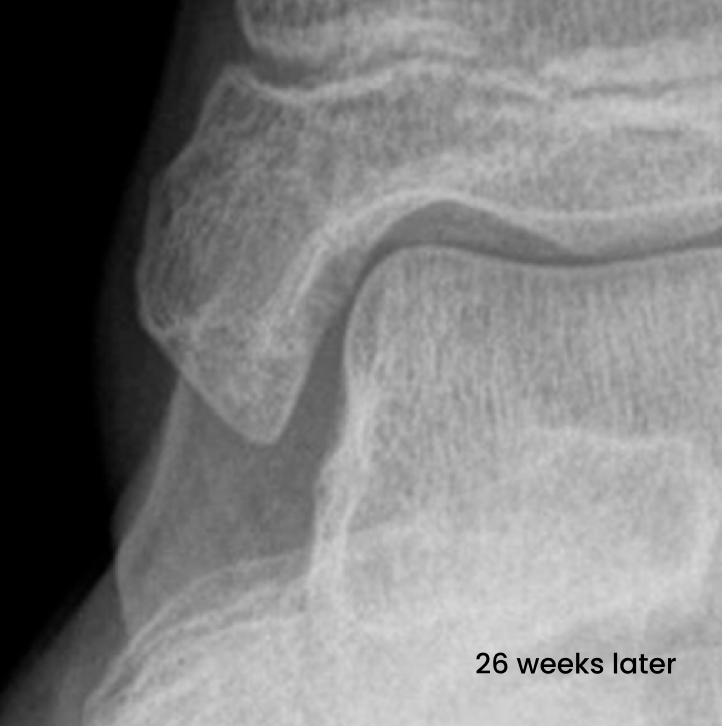
- From Intra Operative to 26 weeks
- Longstanding reliability
- Cost-effective solution
For professionals
ActivaNail™
| Dimensions Ø 1.5 mm and lengths 10-30 mm and diameter 2.0 mm and lengths 15-30 mm. |
Material PLGA |
| Anatomical locations Foot, ankle, knee, hip, hand, wrist, elbow and shoulder |
Certificates FDA-cleared, CE-approved |
Activa Educational materials
We offer our products across all continents, and you can find the location closest to you here.