Activa Series
Absorbable self-reinforced biopolymer
Activa products are absorbable surgical implant used in fracture treatment, osteotomy, arthrodesis and bone surgery. Activa implants integrate with bone, promote faster recovery, and eliminate secondary surgeries, reducing costs.
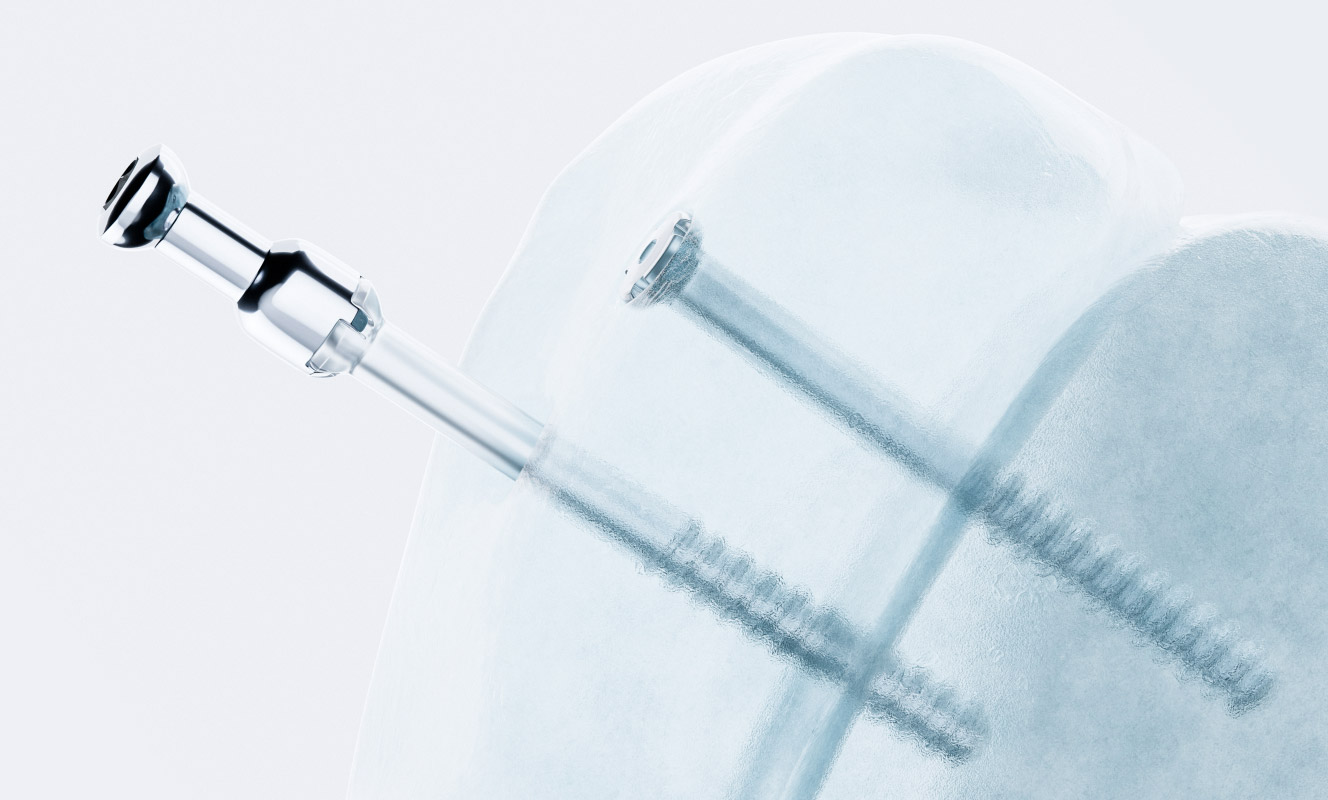

Clinically tested for more than 20 years and used in over 40 countries
Helped over hundreds of thousands of adult and pediatric patients
Excellent in pediatric orthopedics
Fully threaded implants combine safe and absorbable PLGA with Bioretec’s unique self-reinforcement patented technologies
Activa products
This is OUS content
ActivaPin™
ActivaPin™ is an advanced absorbable implant used for the fixation of bone…
ActivaNail™
ActivaNail™ is an advanced absorbable nail implant used in fixation of bone…
Activa Screw™
ActivaScrew™ is an absorbable surgical implant used in fracture treatment,…
ActivaScrew™ Cannulated
ActivaScrew™ Cannulated is an advanced absorbable screw implant used in…
ActivaScrew™ Interference TCP
ActivaScrew™ Interference TCP is an advanced absorbable biocomposite…
Activa IM-Nail™
Activa IM-Nail™ is the world's first fully absorbable elastic nail for…
This is US content
ActivaPin™
ActivaPin™ is an advanced absorbable implant used for the fixation of bone…
ActivaNail™
ActivaNail™ is an advanced absorbable nail implant used in fixation of bone…
Activa Screw™
ActivaScrew™ is an absorbable surgical implant used in fracture treatment,…
ActivaScrew™ Cannulated
ActivaScrew™ Cannulated is an advanced absorbable screw implant used in…
ActivaScrew™ Interference
ActivaScrew™ Interference is an advanced absorbable interference screw implant…

This is OUS content
In Focus
Activa IM-Nail™ - Absorbable Intramedullary Nail
Activa IM-Nail opened a new chapter in pediatric orthopedics with the introduction of the world’s first fully absorbable option for pediatric forearm elastic intramedullary nailing.

This is US content
In Focus
ActivaScrew™ Cannulated -Absorbable Screw
ActivaScrew™ Cannulated is an advanced absorbable screw implant used in fixation of bone fractures, osteotomies, arthrodesis, and osteochondral fractures.
Compare Activa products
This is OUS content
| Absorbable material | Sizes | Anatomical locations | Material | Certificates | Instruments | |
|---|---|---|---|---|---|---|
| ActivaPin™ | Available in diameters 1.5, 2.0, 2.7 and 3.2 mm and lengths 20 – 70 mm | Foot, ankle, knee, hip, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaNail™ | Available in diameter 1.5 and lengths 10-30 mm and diameter 2.0 and lengths 15-30 mm | Foot, ankle, knee, hip, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaScrew™ | Available in diameters 2.0, 2.7, 3.5 and 4.5 mm and lengths 20 – 90 mm | Foot, ankle, knee, hip, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaScrew™ Cannulated | Available in diameters 3.5, 4.0 and 4.5 mm and lengths 20 – 90 mm | Foot, ankle, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaScrew™ Interference TCP | Starts from 4 mm, the largest being 11 mm in diameter | Knee, foot, ankle, elbow, shoulder and hand | PLGA filled β-tricalcium phosphate (TCP) | CE-approved | Open Instrument guide | |
| Activa IM-Nail™ | Available in diameters 2.0, 2.7 and 3.2 mm and lengths 200, 300 and 400 mm | Forearm | PLGA and osteoconductive tricalcium phosphate (β-TCP) marker in the tip | CE-approved | Open Instrument guide |
This is US content
| Absorbable material | Sizes | Anatomical locations | Material | Certificates | Instruments | |
|---|---|---|---|---|---|---|
| ActivaPin™ | Available in diameters 1.5, 2.0, 2.7 and 3.2 mm and lengths 20 – 70 mm | Foot, ankle, knee, hip, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaNail™ | Available in diameter 1.5 and lengths 10-30 mm and diameter 2.0 and lengths 15-30 mm | Foot, ankle, knee, hip, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaScrew™ | Available in diameters 2.0, 2.7, 3.5 and 4.5 mm and lengths 20 – 90 mm | Foot, ankle, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaScrew™ Cannulated | Available in diameters 3.5, 4.0 and 4.5 mm and lengths 20 – 90 mm | Foot, ankle, hand, wrist, elbow and shoulder | PLGA 85/15 | FDA-cleared and CE-approved | Open Instrument guide | |
| ActivaScrew™ Interference | Available in diameters 4-10 mm and lengths 10-33 mm | Knee, foot, ankle, elbow, shoulder and hand | PLGA 85/15 | FDA-cleared | Open Instrument guide |

Activa for Patients and Caregivers
Clinically proven for adult and pediatric patients, Activa eliminates the need for implant removal surgery, supporting a smoother and more efficient healing process.

Activa for Professionals
Activa implants have earned the trust of professionals worldwide as a proven, safe alternative to traditional implants.
Contact us
Are you interested in Activa products or would you like to know more about them?
