ActivaScrew™ Interference TCP -Biocomposite Interference Screw
ActivaScrew™ Interference TCP is an advanced absorbable biocomposite interference screw for fixation of tissue, including ligament or tendon to bone, or a bone-tendon to bone in the presence of appropriate immobilization or controlled mobilization. Interference fixation is appropriate for surgeries of knee, shoulder, elbow, ankle, foot and hand/wrist. Complete absorption takes place within approximately two years, thus eliminating the need for implant removal surgery.
Individually sterile-packaged.
Enhanced tissue attachment and new bone formation with osteoconductive β-TCP and optimized TCP particle size.
Excellent in pediatric orthopedics.
Biocomposite with fully absorbable PLGA mixed with osteoconductive β-tricalcium phosphate (TCP).
For fixation of tissue, including ligament or tendon to bone, or a bone-tendon to bone in the presence of appropriate immobilization/controlled mobilization
ActivaScrew Interference TCP is an implant with excellent mechanical properties created by the tailored material and the proprietary manufacturing technique. The implant provides excellent strength retention over the bone healing period.
The ActivaScrew Interference TCP is manufactured from absorbable, self-reinforced PLGA filled with β-TCP with patented Self-Locking SL™ technology. β-TCP is recognized as an osteoconductive ceramic and it offers exceptional biocompatibility with natural bone components. The technology enables the implant’s diameter to expand 1-2% in the physiological environment and fixes the implant firmly to the bone. Product range starts from the world’s smallest biocomposite screw and it offers 30% more pull-out strength than the market leader due to the self-reinforcement technology.
Patient Benefits
Patented Self-Locking SL™ Technology
ActivaScrew Interference TCP’s patented Self-Locking SL™ technology enables 1–2% expansion under human conditions, enhancing stability and compressing grafts tightly against the drill canal for up to 8–12 weeks postoperatively.
Absorption Within Approximately 2 years
ActivaScrew Interference TCP is absorbable and the implant degrades slowly in the span of approximately two years transferring the stress to the healing bone and assisting in the healing process.
Faster healing
The absorption of ActivaScrew Interference TCP eliminates the need for removal surgery, reducing the risk of implant-related long-term complications and facilitating a smoother healing process.
Indication Examples of Activa Series
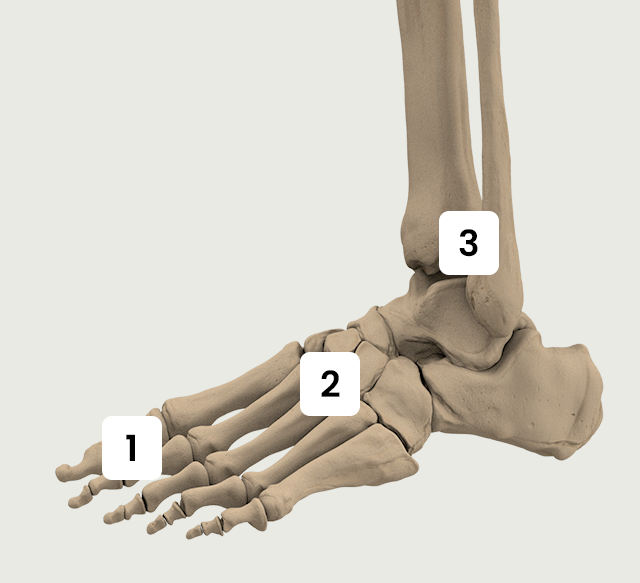
Foot & Ankle
The Activa series applies for fractures and orthopedic conditions in foot and ankle.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
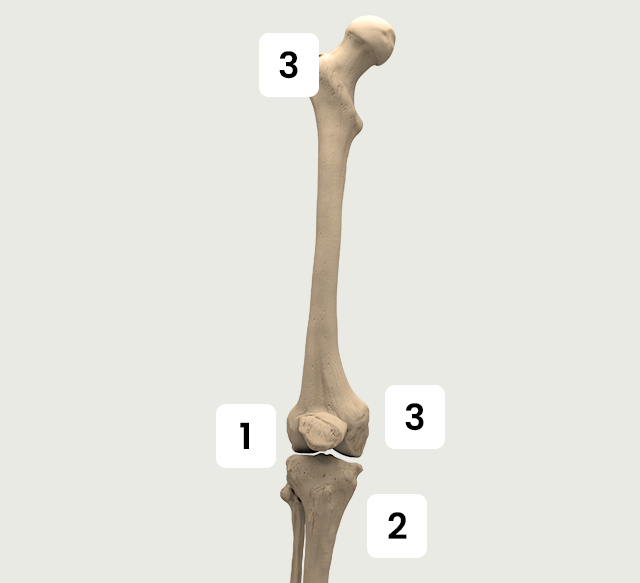
Knee
The Activa series applies for fractures and orthopedic conditions in knee.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
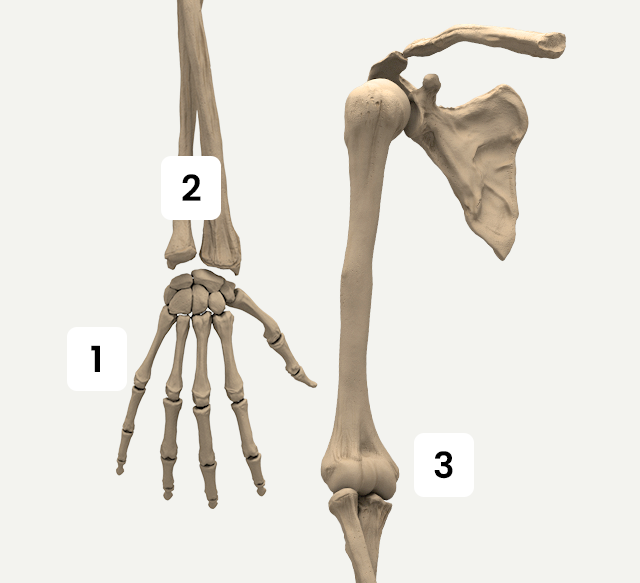
Upper Extremity
The Activa series applies for fractures and orthopedic conditions in Upper Extremity.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Pediatric
The Activa implants are clinically tested for over 20 years, are used in more than 40 countries, and have helped hundreds of thousands of adult and pediatric patients.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Activa -The Difference
Medial malleolus fracture of a 12-year old girl with absorbable ActivaScrew™ LAG
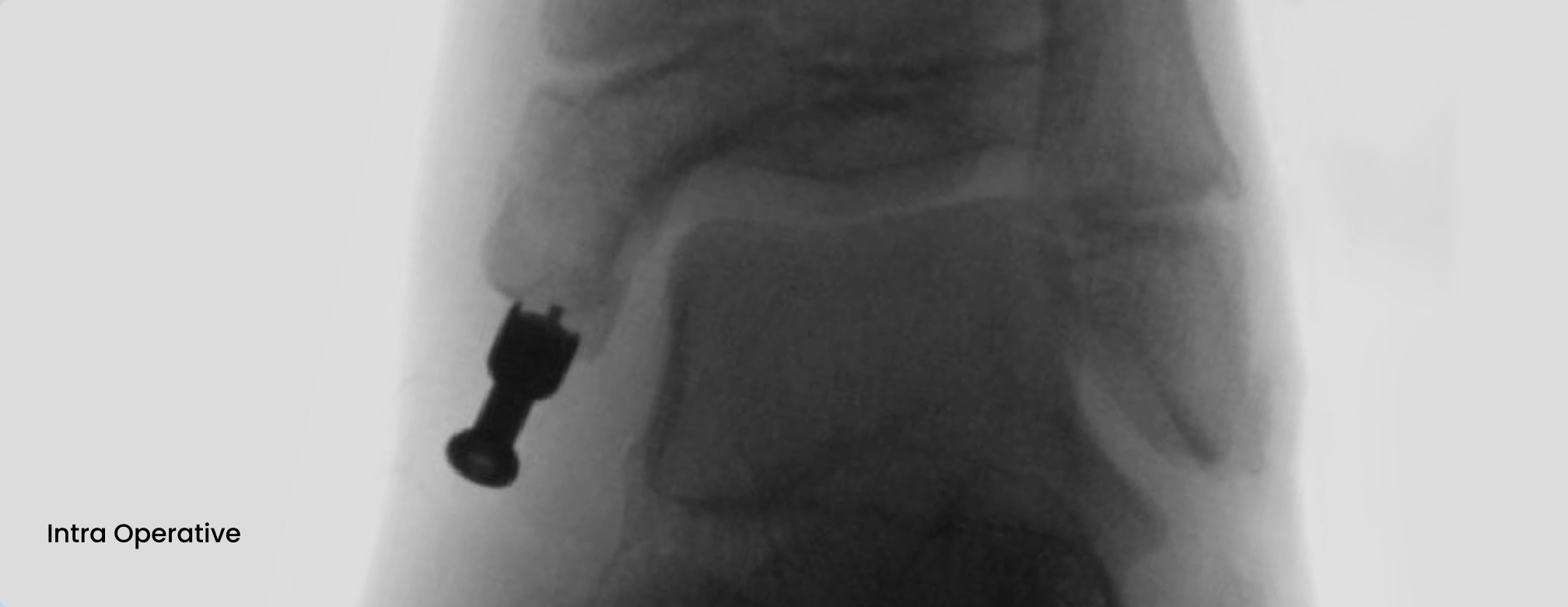
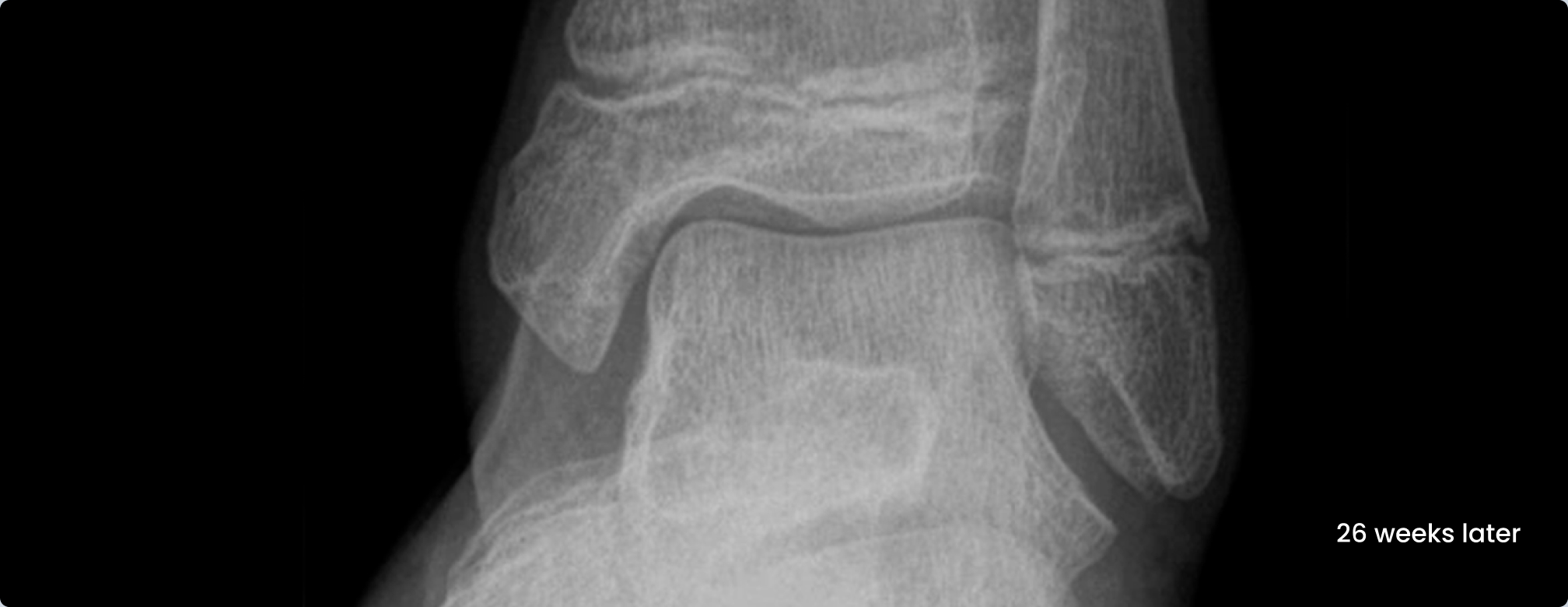
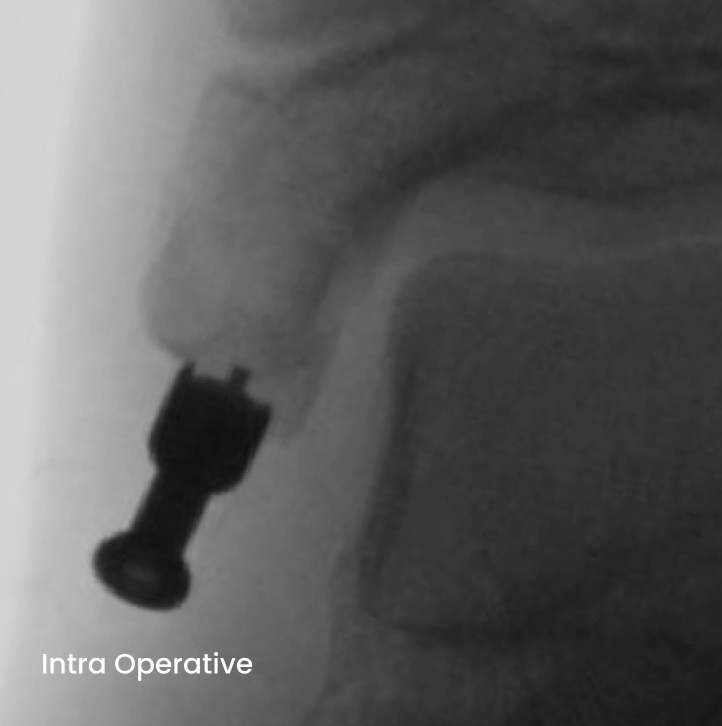
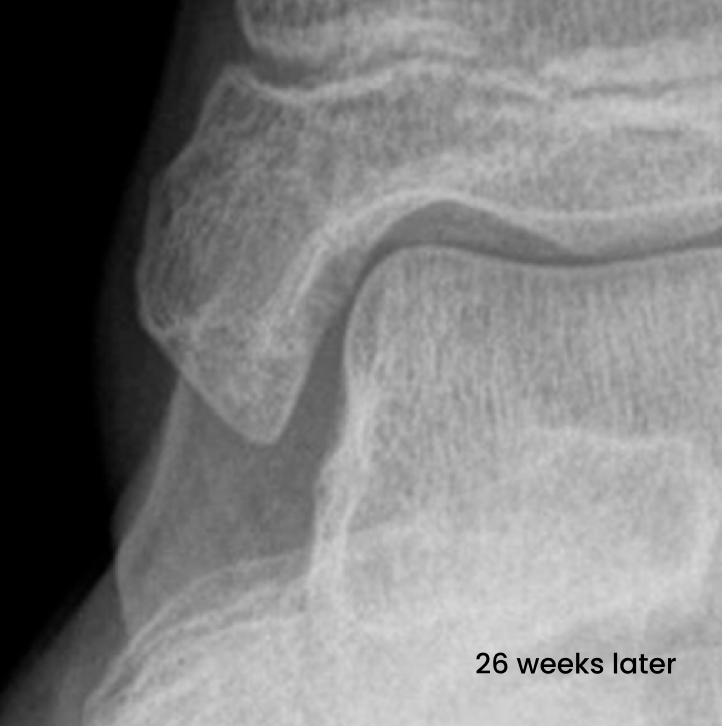
- From Intra Operative to 26 weeks
- Longstanding reliability
- Cost-effective solution
For professionals
The ActivaScrew Interference TCP™
| Dimensions Sizes start from 4 mm, the largest being 11 mm in diameter |
Material Absorbable self-reinforced poly(L-lactide-co-glycolide) (PLGA) filled β-tricalcium phosphate (TCP) |
| Anatomical locations Knee, foot, ankle, elbow, shoulder and hand |
Certificates CE-approved |
The ActivaScrew Interference TCP™ documents
The ActivaScrew Interference TCP™ Product Sheet
Activa Educational materials
We offer our products across all continents, and you can find the location closest to you here.