ActivaScrew™ -Absorbable Screw
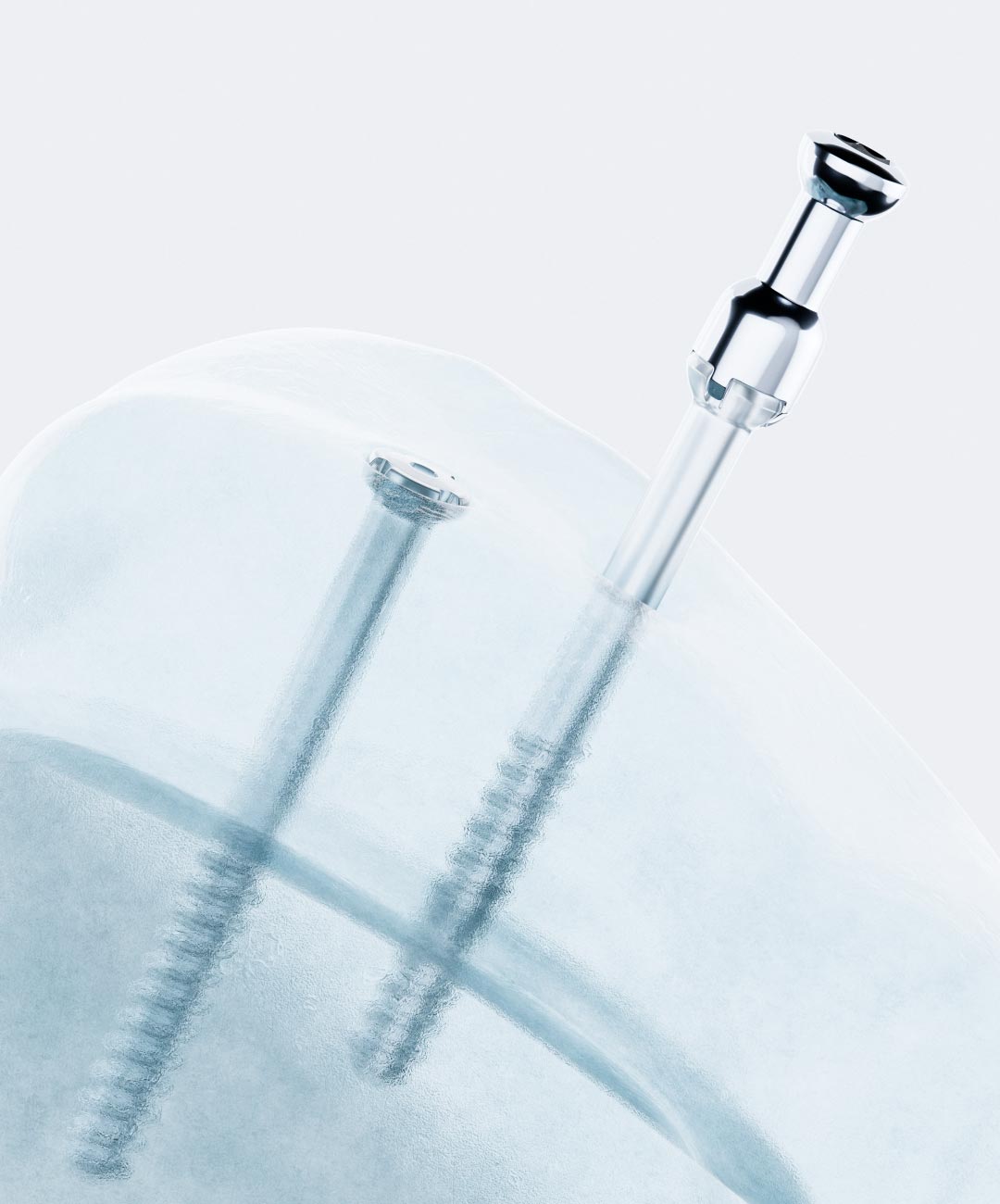
ActivaScrew is an absorbable surgical implant for fixation of bone fractures, osteotomies, arthrodesis, bone grafts and osteochondral fractures of upper and lower extremities in the presence of appropriate immobilization. ActivaScrew material is replaced by bone in approximately two years and eliminates the need for removal surgery.
Individual sterile-packaged and easy insertion with removable metallic insertion adapter.
Auto-Compression™ technology for additional compression.
Excellent also in pediatric orthopedics.
Strong track record of safe use.
This is US content
ActivaScrew™ -Absorbable Screw
ActivaScrew is an absorbable surgical implant for fixation of bone fractures, osteotomies, arthrodesis, bone grafts and osteochondral fractures of upper extremity, ankle and foot in the presence of appropriate immobilization. ActivaScrew material is replaced by bone in approximately two years and eliminates the need for removal surgery.
Individual sterile-packaged and easy insertion with removable metallic insertion adapter.
Auto-Compression™ technology for additional compression.
Excellent also in pediatric orthopedics.
Strong track record of safe use.
For fixation of bone fractures, osteotomies, arthrodesis, bone grafts and osteochondral fractures of upper and lower extremities in the presence of appropriate immobilization.
ActivaScrew is an implant with high-strength and stiffness created by the biodegradable material and the manufacturing patented technology. The bending modulus of the material is close to the value of cortical bone which decreases stress shielding in bone.
ActivaScrew implant provides higher strength and stiffness created by the tailored material and the proprietary manufacturing technique. The material’s bending modulus is closer to the value of cortical bone, which eliminates stress shielding in bone.
This is US content
For fixation of bone fractures, osteotomies, arthrodesis, bone grafts and osteochondral fractures of upper extremity, ankle and foot in the presence of appropriate immobilization.
ActivaScrew is an implant with high-strength and stiffness created by the biodegradable material and the manufacturing patented technology. The bending modulus of the material is close to the value of cortical bone which decreases stress shielding in bone.
ActivaScrew implant provides higher strength and stiffness created by the tailored material and the proprietary manufacturing technique. The material’s bending modulus is closer to the value of cortical bone, which eliminates stress shielding in bone.
Patient Benefits
Patented Auto-Compression™ technology
ActivaScrew’s patented Auto-Compression™ technology reduces the risk of unstable fixation. This technology tightens the fracture line and it provides additional support to proper ossification.
Absorbs within approximately 2 years
ActivaScrew degrades slowly in the span of approximately two years slowly transferring the stress back to the bone and in doing so accelerating rehabilitation.
Faster healing
ActivaScrew fully absorbs in the human body eliminating the need for implant removal operation while reducing patient stress and discomfort and by accelerating the healing process.
Indication Examples of Activa Series
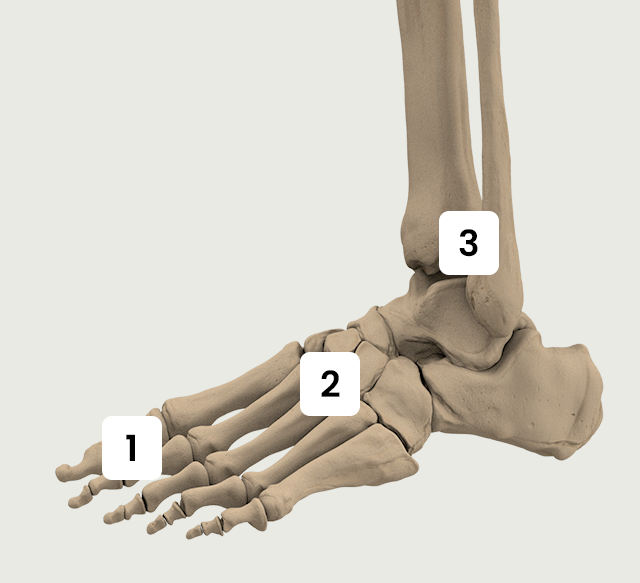
Foot & Ankle
The Activa series applies for fractures and orthopedic conditions in foot and ankle.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
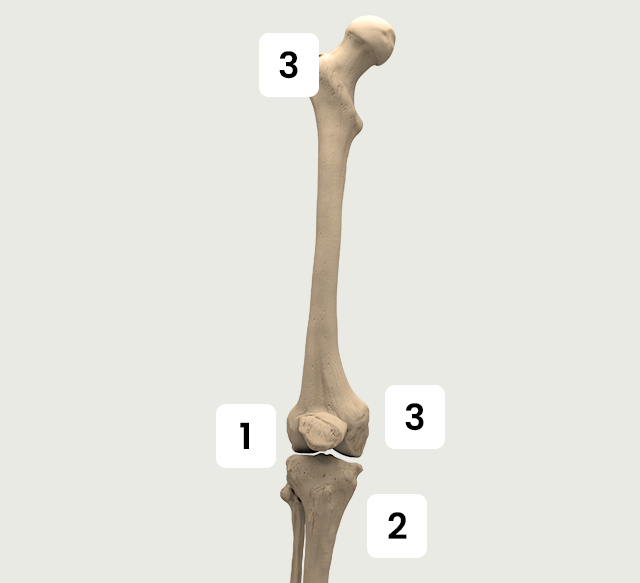
Knee
The Activa series applies for fractures and orthopedic conditions in knee.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
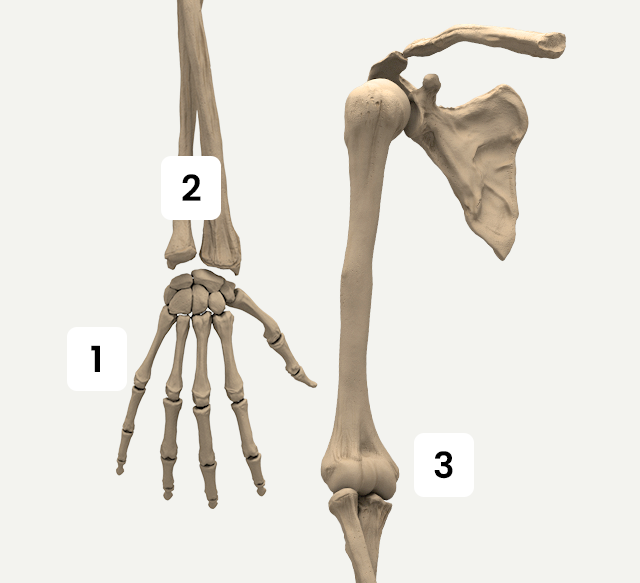
Upper Extremity
The Activa series applies for fractures and orthopedic conditions in Upper Extremity.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Pediatric
The Activa implants are clinically tested for over 20 years, are used in more than 40 countries, and have helped hundreds of thousands of adult and pediatric patients.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Activa -The Difference
Medial malleolus fracture of a 12-year old girl with absorbable ActivaScrew™ LAG
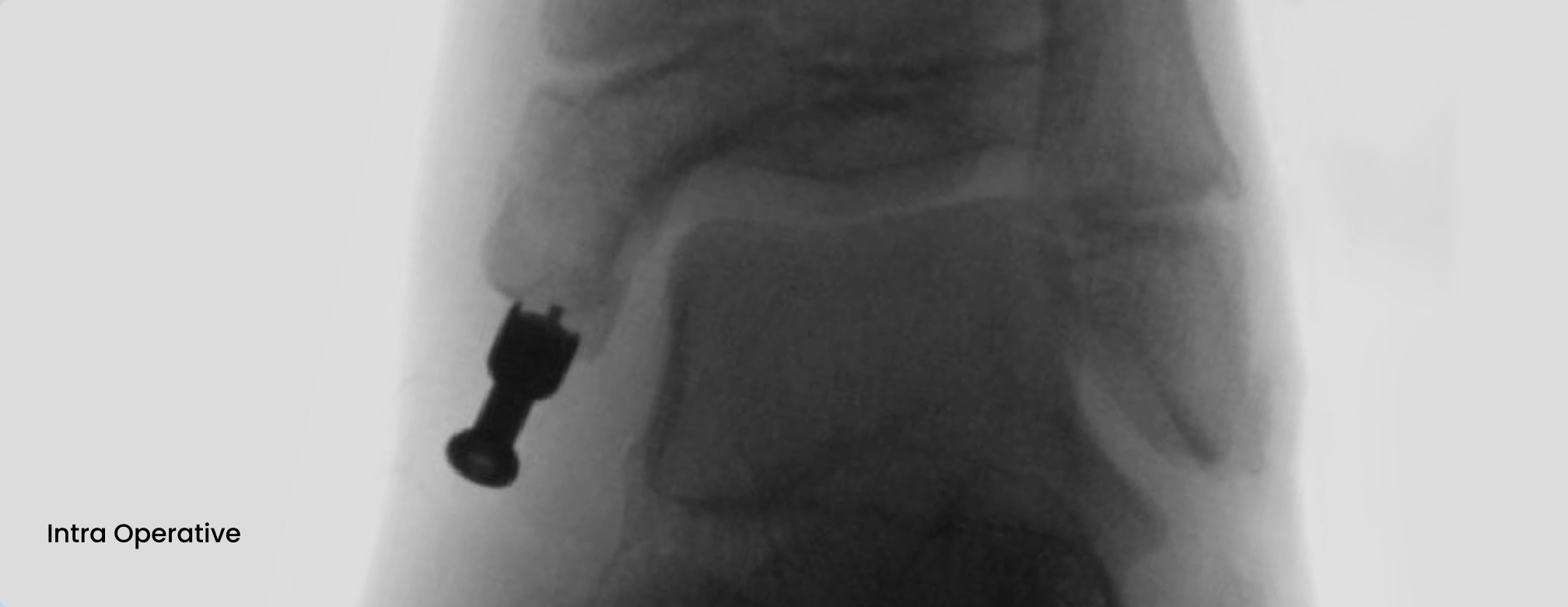
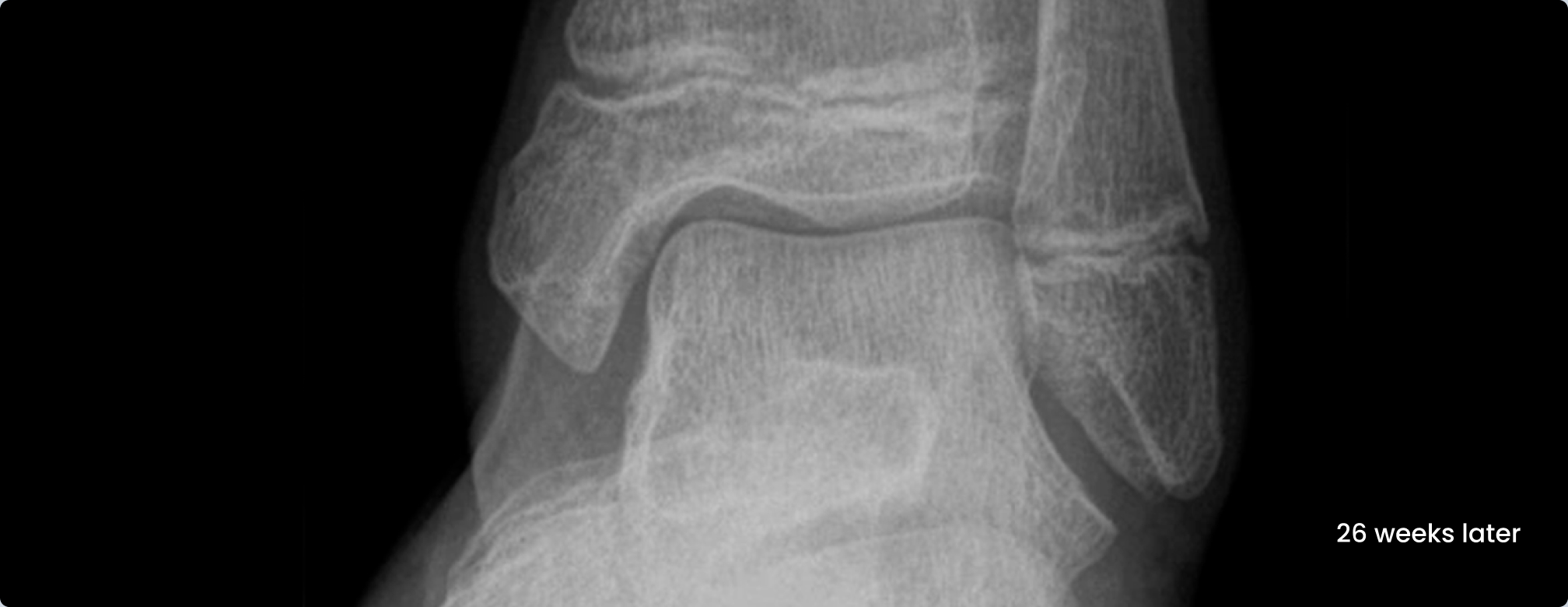
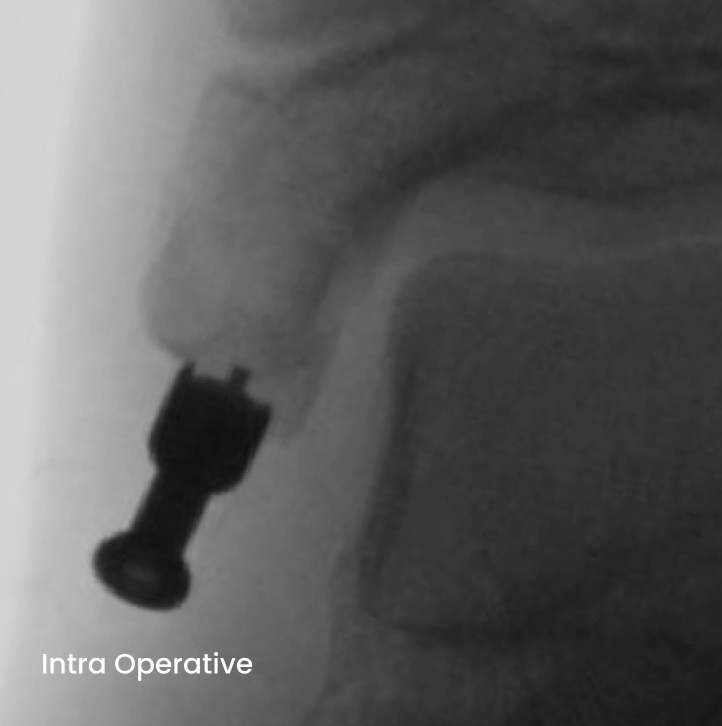
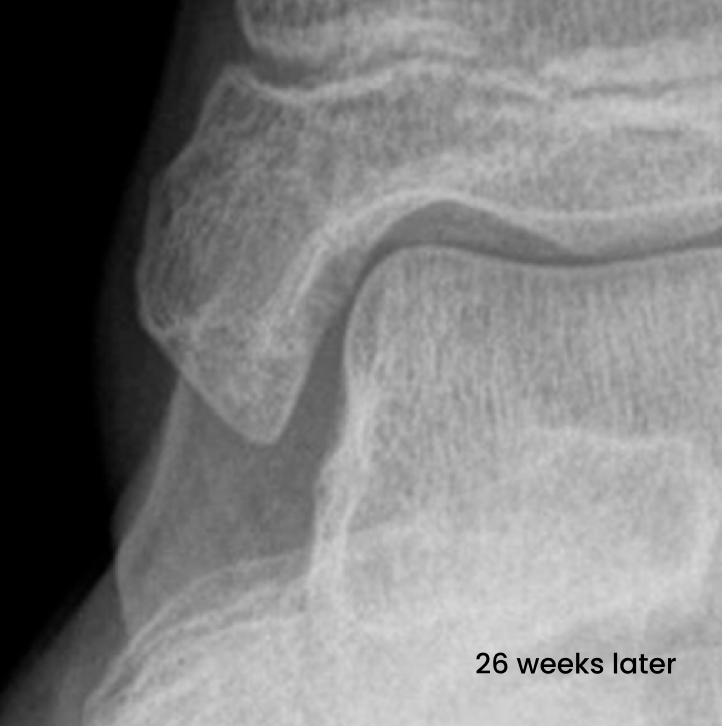
- From Intra Operative to 26 weeks
- Longstanding reliability
- Cost-effective solution
For professionals
ActivaScrew™
| Dimensions Ø 2.0, 2.7, 3.5 and 4.5 mm, lengths 20 – 90 mm |
Material PLGA |
| Anatomical locations Foot, ankle, knee, hip, hand, wrist, elbow and shoulder |
Certificates FDA-cleared, CE-approved |
Activa Educational materials
We offer our products across all continents, and you can find the location closest to you here.
For professionals
This is US content
ActivaScrew™
| Dimensions Ø 2.0, 2.7, 3.5 and 4.5 mm, lengths 20 – 90 mm |
Material PLGA |
| Anatomical locations Foot, ankle, hand, wrist, elbow and shoulder |
Certificates FDA-cleared, CE-approved |
Activa Educational materials
We offer our products across all continents, and you can find the location closest to you here.