ActivaScrew™ Interference -Absorbable Interference Screw
ActivaScrew Interference is an advanced absorbable interference screw implant used in fixation of tissue, including ligament or tendon to bone, or a bone-tendon to bone in the presence of appropriate immobilization or controlled mobilization. ActivaScrew Interference material is replaced by bone in approximately two years and eliminates the need for removal surgery.
Individually sterile-packaged.
Great load-carrying capacity.
Excellent in pediatric orthopedics.
MR safe.
For fixation of tissue, including ligament or tendon to bone, or a bone-tendon to bone in the presence of appropriate immobilization/controlled mobilization. Interference fixation is intended for surgeries of knee, shoulder, elbow, ankle, foot and hand/wrist
The ActivaScrew Interference is a versatile bioabsorbable interference screw designed for secure and biologically integrated fixation. Made from self-reinforced bioabsorbable poly(L-lactide-co-glycolide) (PLGA). Self-reinforcement significantly enhances the interference screw’s mechanical properties, e.g., torsional strength. The ActivaScrew™ Interference naturally degrades approximately within two years, eliminating the need for removal surgery.
Available in sizes ranging from Ø 4 mm to 10 mm, it accommodates diverse surgical needs and is MR-compatible. Its patented Self-Locking SL™ technology enables 1–2% expansion under human conditions, enhancing stability and compressing grafts tightly against the drill canal for up to 8–12 weeks postoperatively.
The screw’s optimized thread profile minimizes graft damage while ensuring strong pull-out strength and homogenous pressure. A center-driven Torx driver design allows for precise insertion, and it is compatible with 0.8 mm or 1.6 mm guide wires. If necessary, reoperation or removal can be performed by drilling through the screw.
With high strength, innovative design, and advanced materials, the ActivaScrew™ Interference ensures reliable and effective fixation for various medical applications.
Patient Benefits
Patented Self-Locking SL™ Technology
ActivaScrew Interference’s patented Self-Locking SL technology minimizes the risk of unstable fixation by enabling the implant’s diameter to expand by 1–2% in a physiological environment.
Absorption Within Approximately 2 years
ActivaScrew Interference is absorbable and the implant degrades slowly in the span of approximately two years transferring the stress to the healing bone and assisting in the healing process.
Faster healing
The absorption of ActivaScrew Interference eliminates the need for removal surgery, reducing the risk of implant-related long-term complications.
Indication Examples of Activa Series
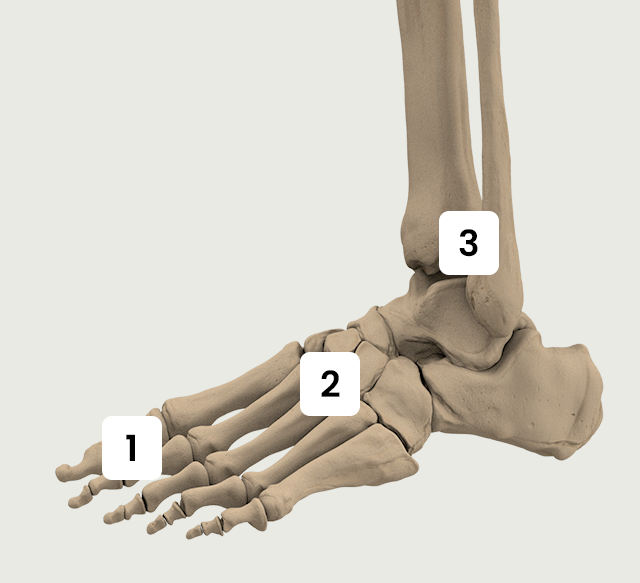
Foot & Ankle
The Activa series applies for fractures and orthopedic conditions in foot and ankle.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
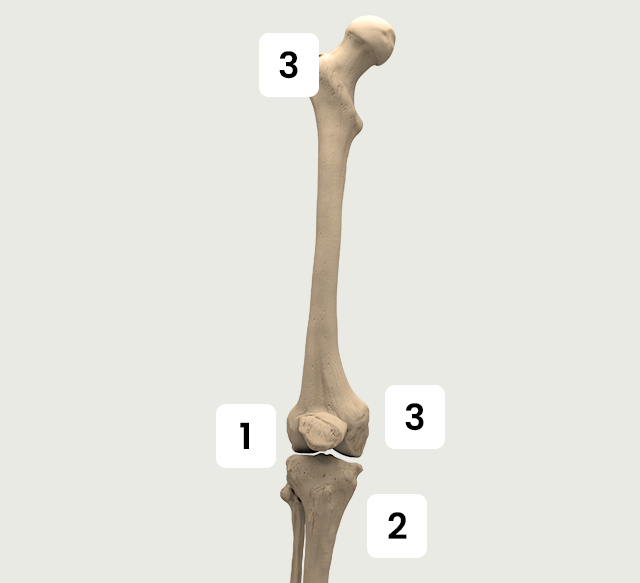
Knee
The Activa series applies for fractures and orthopedic conditions in knee.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
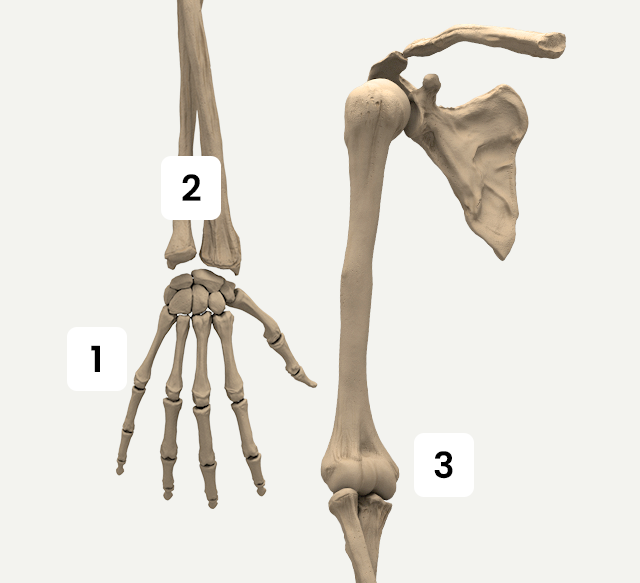
Upper Extremity
The Activa series applies for fractures and orthopedic conditions in Upper Extremity.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Pediatric
The Activa implants are clinically tested for over 20 years, are used in more than 40 countries, and have helped hundreds of thousands of adult and pediatric patients.
Availability of products and their approved intended uses and indications may vary by region. For details, please contact Bioretec customer service.
Activa -The Difference
Medial malleolus fracture of a 12-year old girl with absorbable ActivaScrew™ LAG
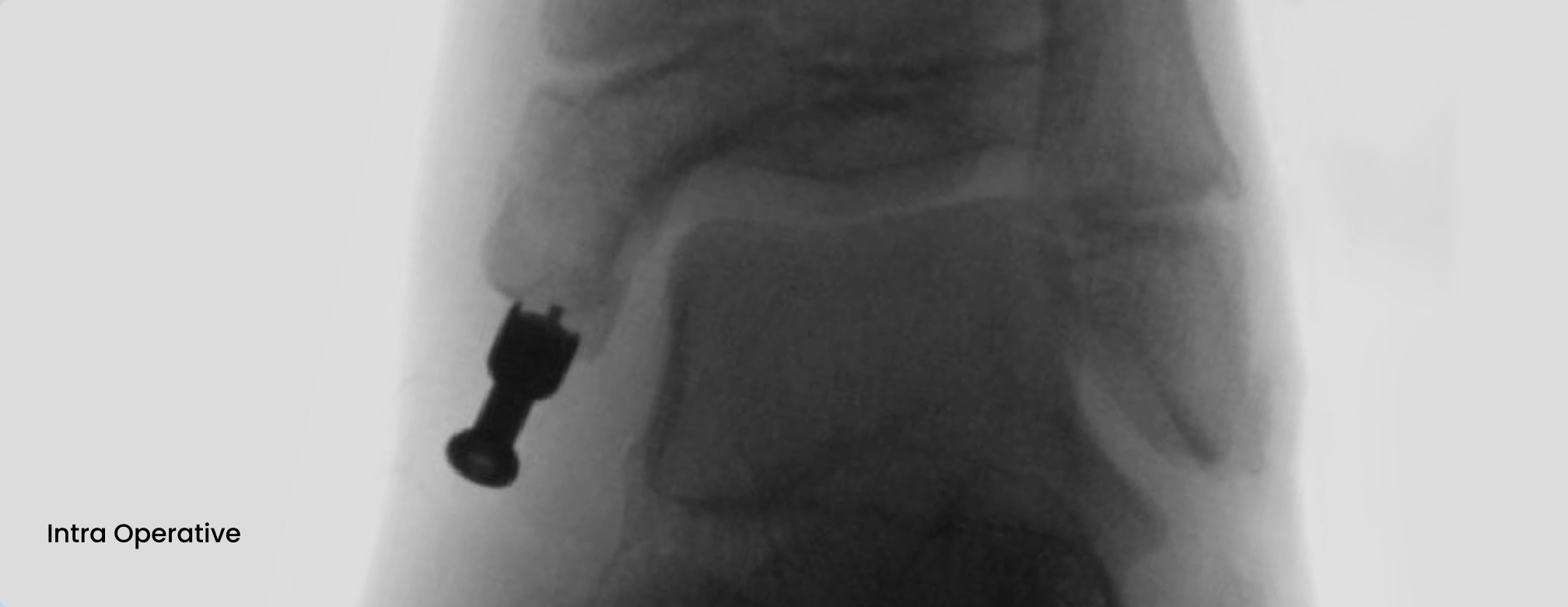
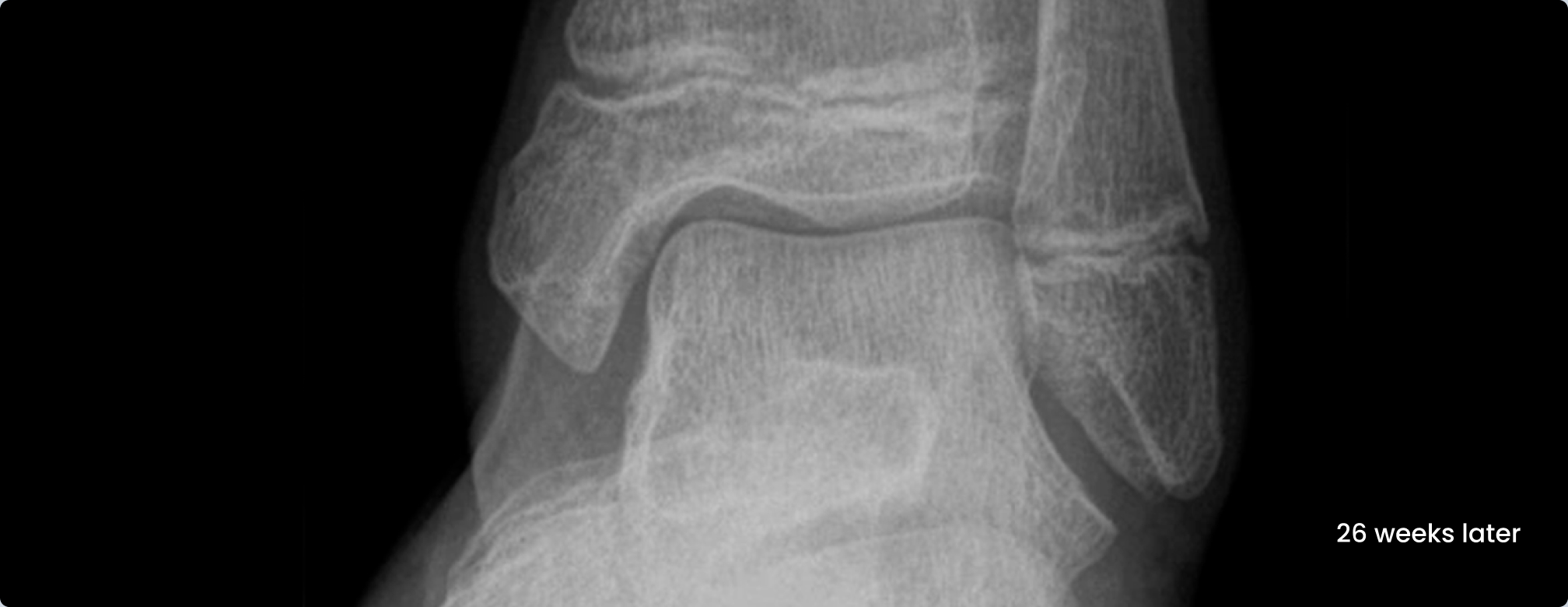
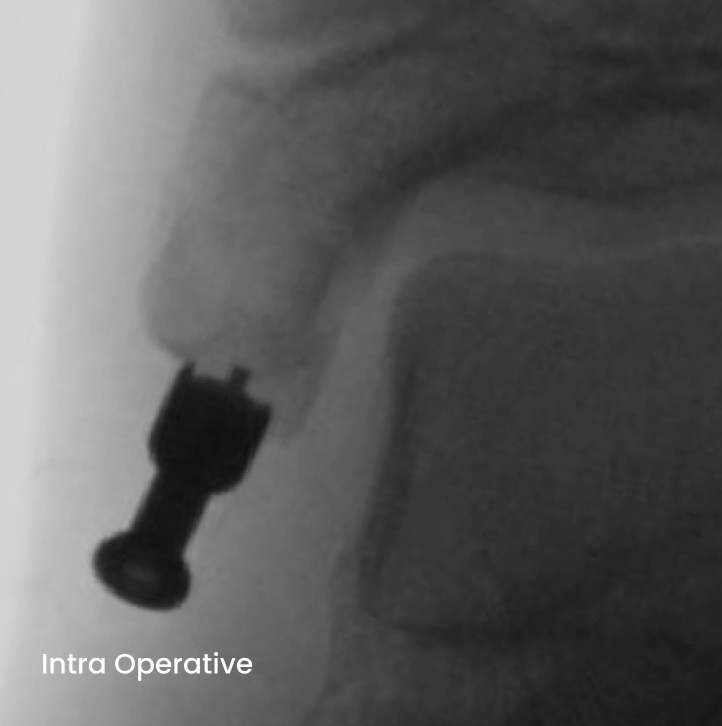
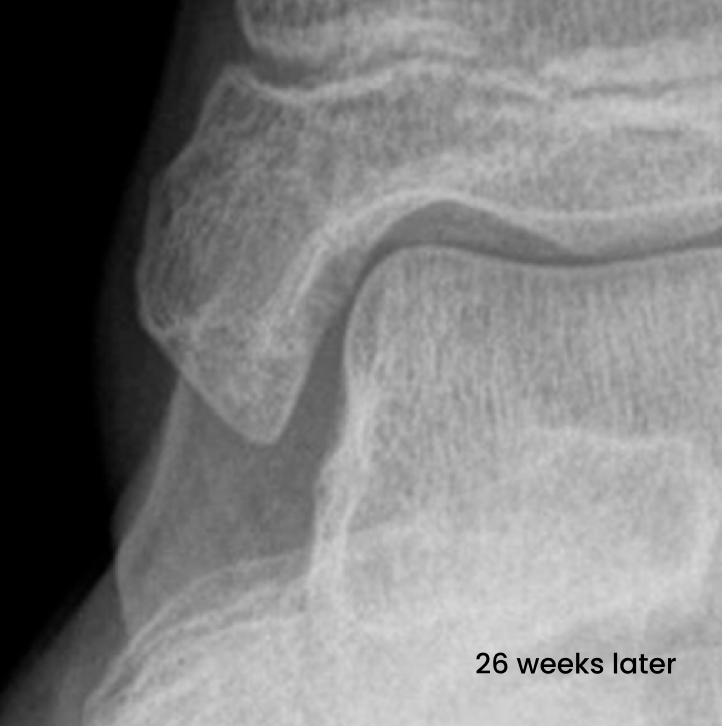
- From Intra Operative to 26 weeks
- Longstanding reliability
- Cost-effective solution
For professionals
The ActivaScrew Interference™
| Dimensions Ø 4-10 mm and lengths 10-33 mm. |
Material PLGA |
| Anatomical locations Knee, foot, ankle, elbow, shoulder and hand |
Certificates FDA-cleared |
The ActivaScrew Interference TCP™ documents
The ActivaScrew Interference TCP™ Product Sheet
Activa Educational materials
We offer our products across all continents, and you can find the location closest to you here.